A throwback in honor of the 2016 Nobel Prize in Chemistry
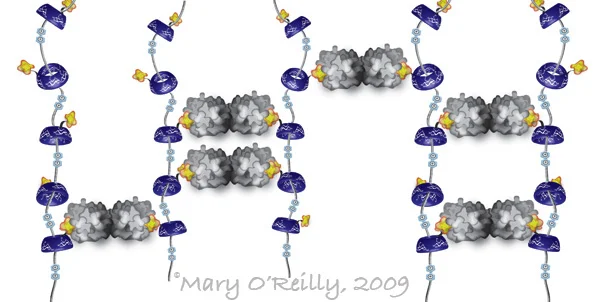
When I was a postdoc in Jim Paulson's lab studying multivalency in protein-carbohydrate interactions, I was invited to the Frie Universitat in Berlin to give two talks, one about multivalency in nature and chemical methods to mimic it, and one specific to my own research. I made the illustration below for the former, to describe a self-assembled pseudopolyrotaxane, often described as the molecular equivalent of beads on a string. It was developed in the lab of Fraser Stoddard, one of the three winners of the Nobel Prize in Chemistry that was just announced. In this collaboration with Linda Baum's group, they built a scaffold of the pseudopolyrotaxane that multivalently displayed a carbohydrate ligand (in yellow), and precipitated its dimeric cognate lectin, galectin (in gray), by forming cross-links like you see in the illustration. The circles you see in between the "beads" are positive charges that act as speed bumps, to stall the beads from just falling off of the string. I remember thinking that this was incredibly cool, and in retrospect apparently just the kind of ingenuity that puts you in line for a Nobel Prize. Looking back on this illustration reminds me of how immensely satisfying I found it to create all of these new illustrations for my talks. I was just on the cusp of letting go of the idea of an academic career in favor of, for lack of a less cliché phrase, following my dream. But at the time I was my only client, and I didn't pay well. Somehow I didn't enjoy it any less.