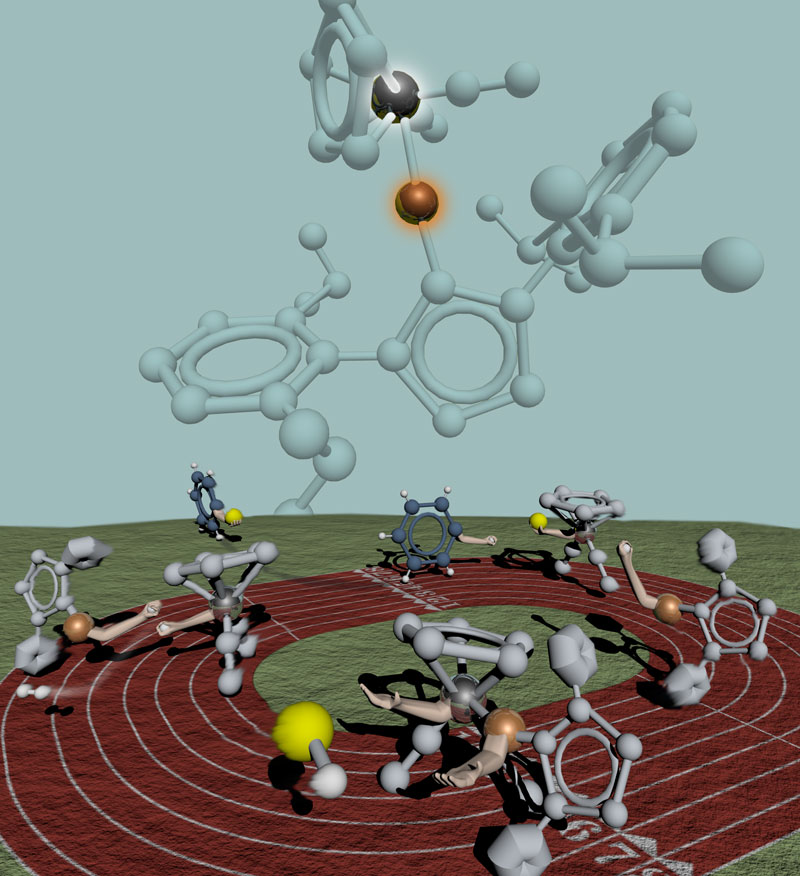
Passing the boron
I'm working on a new project that is, again, super secret until publication, but I can show this "old" one now, the teaser for which I posted last. The bimetallic catalyst shown at the top is capable of efficiently borylating aryl groups as demonstrated in this relay. First the catalyst breaks the B-H bond and breaks apart itself. The yellow boron (attached to hidden "R" groups) then gets traded for a H with benzene. As the borylated benzene leaves the track, the two halves of the catalyst, both now toting H's, come together to reform their bond and make diatomic hydrogen in the process.
As a footnote let me add that atoms do not have arms. They have electrons. Which means that you cannot know both the position and momentum of the arms in this illustration. Which is a lot like how I dance.